Focused Ultrasound Neuromodulation
General information
Overview
- See the FUN21 abstract booklet:
including more details on poster sessions, oral and poster abstracts, more on the conference hall, and a message from the programme committee.
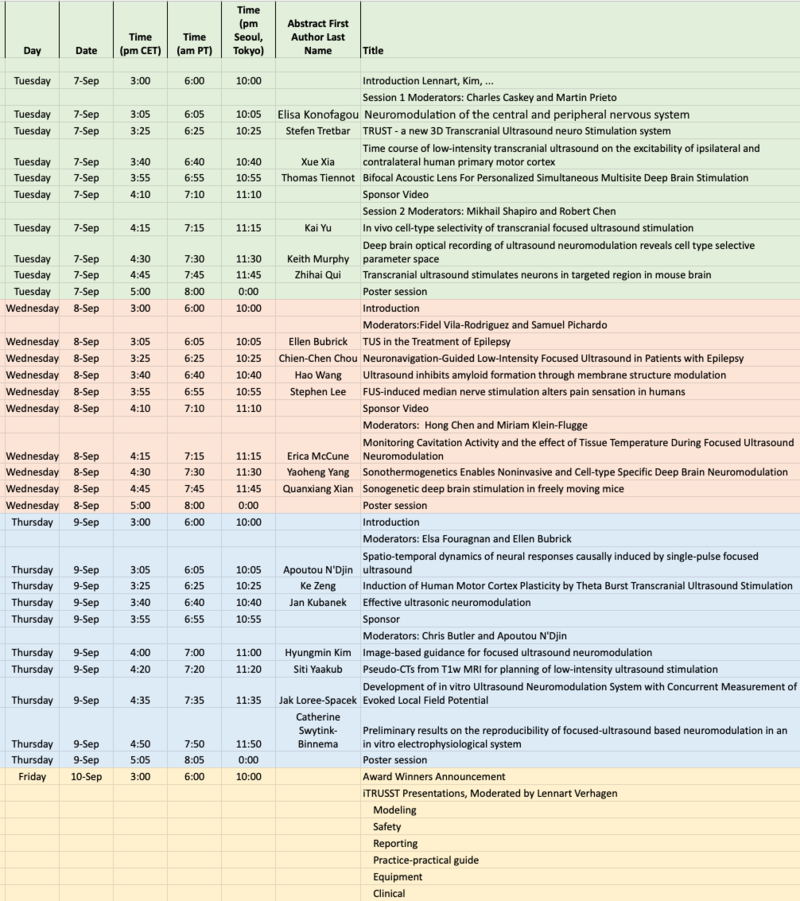
- 7-9th September
- 2 hours of oral talks (6 am PT - 8 am am PT) (3 pm CET—5 pm CET)(11 pm - 1 am S.Korea/Japan)
- 2 hours of posters (8 am PT - 10 am am PT) (5 pm CET—7 pm CET)(1 am - 3 am S.Korea/Japan)
- 10th September (6 am PT - 9 am am PT) (3 pm CET—6 pm CET)(11 pm - 2 am S.Korea/Japan)
- iTRUSST presentation
4 themes and keynote speakers, invited talks and posters presentations
Clinical Invited Presentation: Ellen Jeanne Bubrick, M.D
Neuroscience Invited Presentation: Prof. Elisa Konofagou
Technical Invited Presentation: Dr. Hyungmin Kim
Biomechanisms Invited Presentation: W. Apoutou N'Djin, PhD
---------------------------------------------------
Key dates
| September 7-10, 2021 | symposium |
| September 14-16, 2021 |
educational course |
Symposium Scientific Programme
Posters
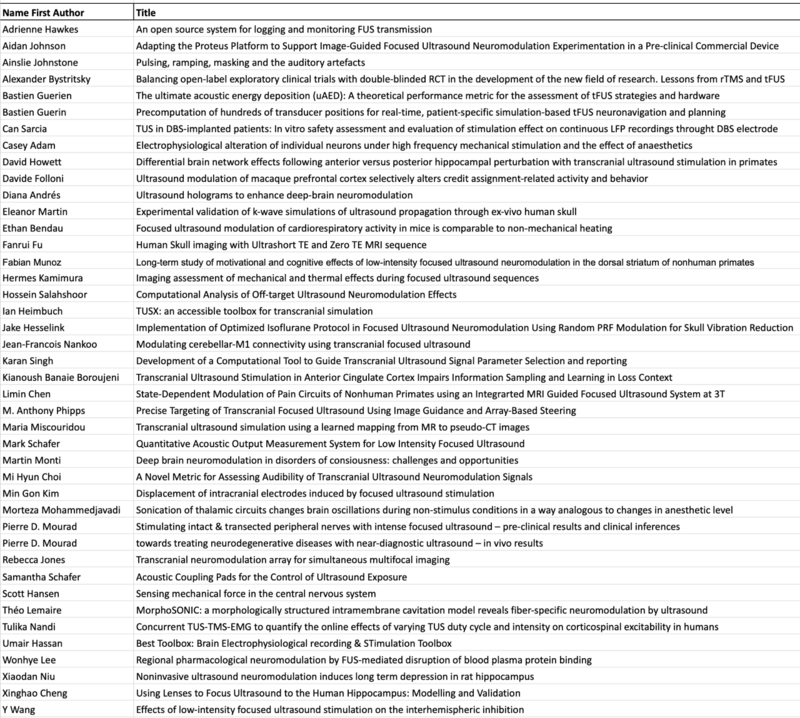
The deadline for poster submission is August the 1st!
Please use the following template for your abstract submission:
Save it as a pdf with the correct filename: NameFirstAuthor_FUN2021.pdf.
Please email fun.conference2021@gmail.com for your abstract submission
Information for presenters. All oral presentations will be done on zoom. The link to the zoomroom will be forthcoming in an email closer to the date of the conference. The format will be a webinar format. All presenters will be invited to be panelists. The audience attendees will not be seen or heard, but they will be able to post questions. Moderators will introduce the speakers, read questions posted by the audience, and ask the questions of the speakers. Abstracts will be published in an abstract book and available online. Late submitted poster abstracts will be added late, but a hard cutoff of Aug 27 for poster abstracts and upload of the poster itself is required in order to allow for adequate gather.town organization. Presentations will be on a private YouTube channel and viewable for 4 weeks after the conference. All poster abstracts and posters must be submitted to fun.conference2021@gmail.com by Aug 27 in order to allow time for upload to gather.town. Posters should be presented such that images and the title are large to allow for quick viewing when people walk by. The main conclusions/take home messages should also be large. Text and methods can be smaller so that viewers who are interested can zoom in on their own. Posters should be 16x9 size. Please check back with regards to whether it should be in PDF/PNG/JPEG format (we need to check on gather.town).
Please note that FUN2021 does not claim copyright on your abstract, poster, or presentation. The copyright remains with the creator, or otherwise current copyright owner. We cannot accept plagiarism or violation of copyright in the submitted or presented material. It is the responsibility of the presenter to ascertain that the necessary permission is in place.
We thank our scientific committee for reviewing the abstracts' suitability and interest to the audience prior to acceptance, in the following categories:
Biomechanisms
Charles Caskey
Martin Prieto
Hong Chen
Mikhail Shapiro
Technical
Samuel Pichardo
Laura Curiel
Ryan Jones
Apoutu N'Djin
Gianmarco Pinton
Neuroscience
David Howett
Miriam Klein-Flugge
Elsa Fouragnan
Jerome Sallet
Julien Claron
Clinical
Chris Butler
Ellen Bubrick
Fidel Vila-Rodriguez
Robert Chen
To register please use the following link :
https://forms.gle/uebPXVy4jTGJz3fM8
Registration fees are 10euros for undergraduate, graduate, PhD students and Postdoctoral Researcher.
Registration fees are 25euros for senior staff, PIs and non-academic attendees.
Registration is mandatory but free for the educational course.
To inquire about registration for the conference or the educational course, please contact fun.conference2021@gmail.com
Information for Oral Presenters:
All oral presentations will be done on zoom. The link to the zoomroom will be forthcoming in an email closer to the date of the conference. The format will be a webinar format. All presenters will be invited to be panelists. The audience attendees will not be seen or heard, but they will be able to post questions. Moderators will introduce the speakers, read questions posted by the audience, and ask the questions of the speakers.
Presentations will be on a private YouTube channel and viewable for 4 weeks after the conference.
Information for Poster Presenters:
Poster sessions will take place in the virtual environment of Gather.Town. Please submit your poster before the deadline of August 27st. When your poster session is scheduled, your avatar should stand in the shaded square in front of your poster. This is a ‘private space’ where your audio/video and poster will only be shared with others standing in the same shaded area (capacity = 9 people).
Please note that your poster will be presented to fit a standard monitor (i.e., 16:9). Therefore, a landscape format poster is strongly recommended. However, Gather.Town allows users to scroll and zoom, so attendees will be able to view your entire poster regardless of its dimensions.
For the poster session we ask that you provide the following documents:
Please email these documents to fun.conference2021@gmail.com
1) Poster in PDF format
Please provide a high-quality .pdf file of your poster2) Poster in .jpg or .png format
Please provide as high quality as possible. The minimum width is 1000 px (26.46 cm) and the minimum height is 600 px (15.88 cm). Please do not use a transparent background for your image.3) A preview ‘thumbnail’ document (.jpg/.png)
Please provide an image that can be used as a ‘thumbnail’ or ‘preview’ of your poster. For example, you can downsize your poster to around half the original image size.
Awards
There will be prizes for the best trainee presentations. All trainees are eligible (students, postdocs, residents, fellows).
More information to come soon.
Trainee Raffle/Scavenger Hunt
We will have a raffle for prize that will be announced on Friday Sept 10 at the start of the FUN / iTRUSST meeting. Entry into the raffle is for registered trainees who have successfully completed the scavenger hunt.
Successful completion includes sending an email by Thursday Sept 9 12 pm CET/ 2 pm PT to fun.conference2021@gmail.com with screen captures of yourself meeting the following 10 people:
- two people from the organizing committee
- three sponsor booths representatives
- someone from Asia, North America, and Europe
- a faculty and a trainee
We will bring together researchers developing and using ultrasound stimulation as a tool for cognitive neuroscience and clinical applications. This symposium will have a strongly integrative and translational approach from mechanisms, to neural circuits, to applications, from engineering, to neuroscience, to the clinic.
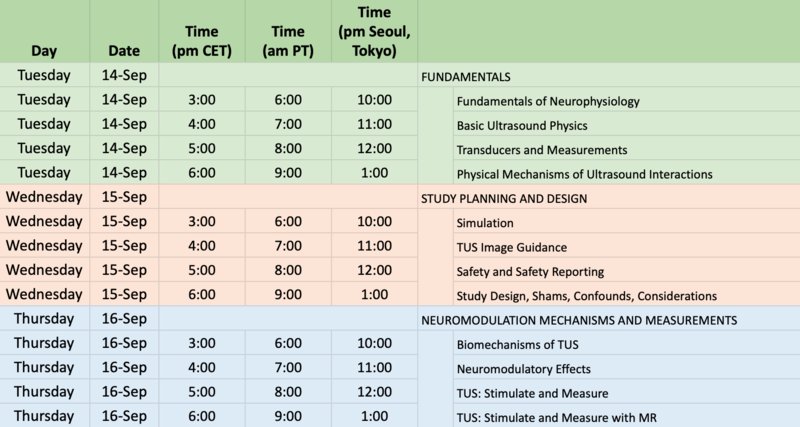
14-16th September: Educational course (free) on TUS
- 3 days / 4 hours per day
- Tutorials and educational courses (45 min each) + 15min Q&A covering a large range of topics, from the basic use of TUS to more advanced ultrasound physics concepts and measurements
----------------------------------------------------------
Oral presentations will be done in a Zoom Webinar format.
Poster sessions will be on gather.town.
We will see you soon!
Scientific Symposium - Sept 7-10 2021
Educational course on TUS - Sept 14-16 2021
speakers
Talk: Neuromodulation of the central and peripheral nervous system
More information about Elisa
Talk: Image-based guidance for focused ultrasound neuromodulation
More information about Hyungmin
Talk: Spatio-temporal dynamics of neural responses causally induced by single-pulse focused ultrasound
More information about Apoutou
organizers / scientific committee
Kim is a Professor at Stanford University. Her lab focuses on ultrasound stimulation in vivo, from the mouse model to humans. Her interests range from technical aspects to biomechanisms to applications. Her toolbox relies heavily on imaging, from GCaMP imaging in mice to MR imaging and guidance in humans
Charles is an Associate Professor of Radiology and Biomedical Engineering at Vanderbilt University and Medical Center in Nashville, Tennessee. He currently leads the Laboratory of Acoustic Therapy and Imaging at the Vanderbilt University Institute of Imaging Science. His laboratory focuses on developing ultrasound as a tool for neuromodulation, drug delivery, and functional imaging.
Elsa is a Future Leader Fellow (MRC - UKRI), head of the NIBS lab at BRIC and lecturer et the University of Plymouth, UK. She combines neurostimulation and computaional neuroscience to study decision making and learning in healthy adults and patients with psychiatric conditions.
Samuel is an Assistant Professor at the University of Calgary. His research covers the characterization of transcranial ultrasound, exploration of focused ultrasound for neurostimulation applications and the development of software platforms for image-guided therapy.
Jerome is a tenured behavioral neuroscientist at INSERM (Lyon, France). His research focuses on the neurobiological basis of adaptive behavior, with a specific interest in social behaviors. His approach combines comparative neuroanatomical studies and neuroimaging studies in humans and non-human primates.
https://www.sbri.fr/users/jeromesallet
Bradley is a Professor of Biomedical Ultrasound at University College London. His main interests relate to therapeutic ultrasound, and in particular, the development of simulation tools for treatment planning.
Lennart is an Assistant Professor at the Donders Institute, Radboud University (Nijmegen, the Netherlands). His lab develops ultrasonic neuromodulation approaches for human applications, focussing on brain circuits for behavioural control and learning.
Chris is a Clinical Senior Lecturer in Cognitive Neurology at Imperial College London. His work combines neuropsychology, neuroimaging and non-invasive brain stimulation to investigate cognitive disorders in neurological disease, especially dementia and epilepsy.
https://www.neuroscience.ox.ac.uk/research-directory/christopher-butler
Benjamin is a PhD student at the Donders Institute, Radboud University (Nijmegen, The Netherlands). His work focuses on transcranial ultrasonic stimulation of motor and decision making circuits in humans.
















